New Publication in "Science": "Dynamics and mechanism of a light-driven chloride pump"
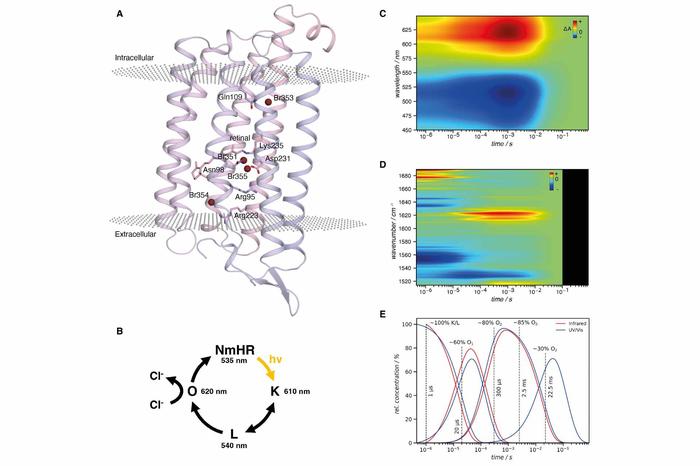
Halide binding sites, photocycle of the rhodopsin NmHR and spectroscopic data
Image Credit: Science (3 Feb 2022); DOI: 10.1126/science.abj6663
An international scientific team associated with the collaborative research center SFB 1078 "Protonation Dynamics in Protein Function" suggested that the charge separation observed in a light driven chloride pump is a fundamental feature for light energy conversion in nature as well as in technology.
News from Feb 14, 2022
In a recent publication in “Science”, researchers from Freie Universität Berlin, ETH Zurich, Paul Scherrer Institute Villigen and the Hebrew University of Jerusalem proposed the ion transfer pathway in bacterial halorhodopsin and discussed the interplay between the driving force created by the protein dipole moment and the control of transport by molecular gates. They provided details on how light energy is converted into kinetic energy for chloride translocation. The resulting charge separation represents a fundamental feature for light energy conversion in nature as well as in technology.
Chloride transport by microbial rhodopsins is an essential process. However, its molecular details, such as the mechanisms that convert light energy to drive ion pumping and ensure the unidirectionality of the transport, have remained elusive yet. The researchers combined time-resolved serial crystallography with time-resolved spectroscopy and multiscale simulations to elucidate the molecular mechanism of a chloride pumping rhodopsin and the structural dynamics throughout the transport cycle.
They traced transient anion binding sites, obtained evidence for how light energy is used in the pumping mechanism, and identified steric and electrostatic molecular gates ensuring unidirectional transport. An interaction with the π-electron system of the retinal supports transient chloride ion binding across a major bottleneck in the transport pathway.
These results allowed the authors to propose key mechanistic features enabling finely controlled chloride transport across the cell membrane in this light-powered chloride ion pump.
About SFB 1078
In the Collaborative Research Center 1078 "Protonation Dynamics in Protein Function", scientists from physics, biology, and chemistry work jointly to identify and understand a new key principal in protein science, namely the control and coordination of complex protein function by protonation dynamics. The spokesperson of the researchcenter is biophysics professor Dr. Joachim Heberle of Freie Universität Berlin.
Keywords
- charge separation
- chloride pump
- chloride transport
- ion pumping
- light energy conversion
- light-driven
- multiscale simulations
- Protonation Dynamics in Protein Function
- publication
- Science
- SFB 1078
- time-resolved serial crystallography
- time-resolved spectroscopy