The active site of metalloproteins - Spectroscopic investigations on catalytic mechanisms
About half of all proteins contain transition metals in their active site. Presently, there is increasing scientific interest in these metal centers, because many important biological processes are catalyzed by protein-bound transition metals. Furthermore, biomimetic approaches lead to interesting perspectives in (bio-)technology. Thus, a new interdisciplinary field of research developed, called bioinorganic chemistry.
In many cases, e.g. membrane proteins, crystallographic determination of the molecules structure cannot be performed with reasonable expenditure. This is especially true if, for elucidation of the catalytic mechanism, the structure of the active site has to be unraveled in many intermediate states of the functional cycle. By X-ray absorption spectroscopy (XAS), determination of the oxidation state of the X-ray absorbing metal atom (position of the absorption edge) and of the adjacent structures (EXAFS effect) becomes feasible. The EXAFS phenomenon (extended X-ray absorption fine structure, EXAFS) allows precise determination of distances between the absorbing metal atom and surrounding atoms of the first coordination spheres. X-ray absorption measurements have been performed by us at the outstation of the European Molecular Biology Laboratories (EMBL) at the Deutsches Elektronen-Synchrotron (DESY) in Hamburg (in cooperation with Dr. W. Meyer-Klaucke), at the European Synchrotron Radiation Facility (ESRF) in Grenoble (in cooperation with Dr. P. Glatzel), at the Berliner Elektronensynchrotron (BESSY, in cooperation with Prof. A. Erko and Dr. F. Schäfers), and at other European synchrotron radiation sources.
| One of our 'specialties' is the orientation of protein particles through a combination of centrifugation techniques and partial dehydration. Such vectorially oriented samples render XALDS measurements feasible (X-ray absorption linear dichroism spectroscopy, XALDS; variation of the angle between the sample and the X-ray's electric field vector). In many cases the achievable resolution of EXAFS measurements is increased by XALDS, and the determination of the spatial orientationa of the metal complex becomes possible. Our aim is to develop this method (regarding preparation and measurement techniques, theory of the effect, evaluation of experimental data) in order to take full advantage of the potential of the XALDS technique. | 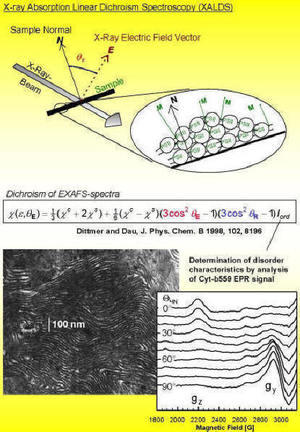 |
Another promising approach is time-resolved XAS. The metalloprotein is driven through its catalytic cycle right at the beamline, thereby allowing 'on-line' measurement of changes in structure and oxidation state of the metal center. Experiments at the European Synchrotron Radiation Facility (ESRF, Grenoble) have been successful (Haumann et al., 2005, Science; 2008, PNAS.).
Numerous other question on the role of metals in biology have been investigated. Spatially resolved XAS measurements of the atomic structure of volcano-shaped manganese deposits in the cell wall of certain green algae have been performed in cooperation with PD C. Plieth (Kiel). The closely cooperating research group of PD M. Haumann at the Physics Dept. of the FU Berlin investigates Ni-Fe and Fe-Fe hydrogenases of various organisms in cooperation with numerous national and international collaboration partners, as well as molybdenum enzymes and Mn-Fe containing ribonucleotide reductases.