FORSCHUNGSTHEMEN
1. Methods for in-situ electron spin resonance spectroscopy - watching membrane proteins in cells and native membranes
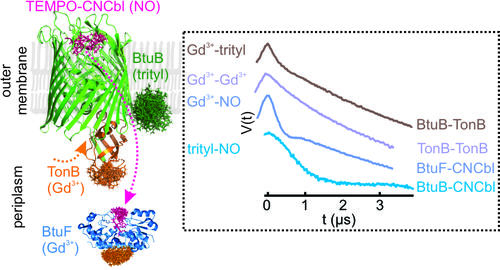
Tripple labeling and distance measurements for heterooligomeric complexes in the native membranes (Ketter S and Joseph B, JACS (2023))
Membrane proteins often transverse through a broad energy landscape and undergo large conformational changes during function. The dream for a structural biologist is to observe them in the cellular environment. Over the past few years, we demonstrated an in-situ pulsed ESR spectroscopy approach (DEER/PELDOR) for observing the structure and conformational changes of outer membrane protein complexes in the native membrane and intact E. coli. This allowed us to observe the structure and dynamics of the cobalamin transport complex (BtuB-TonB), β-barrel assembly machinery (BAM) and the outer membrane lipopolysaccharide translocon LptDE in intact E. coli and isolated native membranes. For this purpose, we employ new spin labels, labeling strategies, sample preparation protocols, and advanced pulse sequences.
Related Publications
- Joseph B (2024). Protein-protein interaction and conformational change in the alpha-helical membrane transporter BtuCD-F in the native cellular envelope.ChemBioChem https://doi.org/10.1002/cbic.202400858
- Ketter S, Gopinath A, Joseph B (2024) Conformational heterogeneity of β-barrel membrane proteins observed in situ using orthogonal spin labels and pulsed ESR spectroscopy. Methods. Mol. Biol., 2778:237-257, 10.1007/978-1-0716-3734-0_15
- Ketter S, Joseph B (2023) Gd(III) - trityl - nitroxide triple labelling and distance measurements in the heterooligomeric cobalamin transport complex in the native lipid bilayers. J. Am. Chem. Soc.,145, 2, 960–966, https://doi.org/10.1021/jacs.2c10080
- Gopinath A and Joseph B (2021) Conformational flexibility of the protein insertase BamA in the native asymmetric bilayer elucidated with ESR spectroscopy. Angew. Chem. Int. Ed., e202113448, https://doi.org/10.1002/anie.202113448
- Kugele A, Ketter S, Silkenath B, Wittmann V, Joseph, B* Drescher M * (2021) In situ EPR Spectroscopy of a Bacterial Membrane Transporter using an Expanded Genetic Code. Chem. Commun., 57, 12980-12983, https://doi.org/10.1039/D1CC04612H
- Ketter S, Gopinath A, Rogozhnikova O, Trukhin D, Tormyshev VM, Bhagryanskaya EG, Joseph B (2021) In situ labeling and distance measurements of membrane proteins in E. coli using Finland and OX063 trityl labels. Chem. Eur. J., 27:2299-2304.https://doi.org/10.1002/chem.202004606
- Joseph B*, Jaumann EA, Sikora A, Barth K, Prisner TF, Cafiso DS (2019) In situ observation of conformational dynamics and protein-ligand/substrate interaction in outer membrane proteins with DEER/PELDOR spectroscopy. Nat. Protoc., 14:2344-2369, https://doi.org/10.1038/s41596-019-0182-2
- Joseph B*, Tormyshev VM, Rogozhnikova OYu, Akhmetzyanov D, Bagryanskaya EG, Prisner TF* (2016) Selective High-Resolution Detection of Membrane Protein-Ligand Interaction in Native Membranes using Trityl-Nitroxide PELDOR. Angew. Chem. Int. Ed., 55, 11538-11542, https://doi.org/10.1002/anie.201606335
- Joseph B*, Sikora A, Cafiso DS* (2016) Ligand-induced conformational changes in a membrane transporter in E. coli cells observed with DEER/PELDOR. J. Am. Chem. Soc., 138, 1844-1847, https://doi.org/10.1021/jacs.5b13382 (highlighted in JACS Spotlights; *corresponding authors)
- Sikora A, Joseph B, Matson M, Staley JR, Cafiso DS (2016) Allosteric Signaling Is Bidirectional in an Outer-Membrane Transport Protein. Biophys J., 111, 1908-1918, https://doi.org/10.1016/j.bpj.2016.09.038
- Joseph B, Sikora A, Bordignon E, Jeschke G, Cafiso DS, Prisner TF (2015) Distance Measurement on an Endogenous Membrane Transporter in E. coli Cells and Native Membranes Using EPR Spectroscopy. Angew. Chem. Int. Ed., 54, 6196-6199, https://doi.org/10.1002/anie.201501086
2. Membrane transport mechanisms
The function of cells depends on transport of diverse molecules across the biological membrane. When the molecules move along a concentration gradient, the chemical potential energy itself could drive the transport. However, membrane transporters often carry substrates against a concentration gradient. In order to overcome the energy barrier, they couple an external energy source with substrate translocation. Depending on the nature of the transporter, the energy may be provided by ATP, electrochemical gradient, or light etc. We study molecular principles underlying energy transduction and conformational dynamics in Type IV and Type VI ATP Binding Cassette (ABC) transporters. Time resolved spectroscopic experiments employing nitroxide labels and endogenous paramagnetic metal centers are employed in these studies.
Related Publications
- Rudolph M, Tampé R, Joseph B (2023) Time-resolved Mn2+–NO and NO–NO distance measurements reveal that catalytic asymmetry regulates alternating access in an ABC transporter. Angew. Chem. Int. Ed., https://doi.org/10.1002/anie.202307091
- Barth K, Rudolph M, Diederichs T, Prisner TF, Tampé R*, Joseph B* (2020) Thermodynamic Basis for Conformational Coupling in an ATP-Binding Cassette Exporter. J. Phys. Chem. Lett., 11:7946-7953. https://doi.org/10.1021/acs.jpclett.0c01876
- Chang YN, Jaumann EA, Reichel K, Hartmann J, Oliver D, Hummer G*, Joseph B*, Geertsma ER* (2019) Structural basis for functional interaction in dimers of SLC26 transporters. Nat. Commun., (*corresponding authors), https://doi.org/10.1038/s41467-019-10001-w
- Barth K, Hank S, Spindler PE, Prisner TF, Tampé R, Joseph B (2018) Conformational Coupling and trans-Inhibition in the Human Antigen Transporter Ortholog TmrAB Resolved with Dipolar EPR Spectroscopy. J. Am. Chem. Soc., 140, 4527–4533, https://doi.org/10.1021/jacs.7b12409
- Bock C, Löhr F, Tumulka F, Reichel K, Würz J, Hummer G, Schäfer L, Tampé R, Joseph B, Bernhard F, Dötsch V, Abele R. Structural and functional insights into the interaction and targeting hub TMD0 of the polypeptide transporter TAPL (2018). Sci. Rep., 8(1):15662, https://doi.org/10.1038/s41598-018-33841-w
- Nöll A, Thomas C, Herbring V, Zollmann T, Barth K, Mehdipour AR, Tomasiak TM, Brüchert S, Joseph B, Abele R, Oliéric V, Wang M, Diederichs K, Hummer G, Stroud RM, Pos KM, and Tampé R (2017) Crystal structure and mechanistic basis of a functional homolog of the antigen transporter TAP. Proc. Natl. Acad. Sci. USA., 114, E438-447, https://doi.org/10.1073/pnas.1620009114
- Joseph B, Korkhov VM, Yulikov M, Jeschke G, Bordignon E (2014) Conformational cycle of the vitamin B12 ABC importer in liposomes detected by double electron-electron resonance (DEER). J. Biol. Chem., 289, 3176-3185, https://doi.org/10.1074/jbc.M113.512178
3. Transenvelope lipid and protein translocation in Gram-negative bacteria
Gram-negative bacteria have become increasingly resistant to available antibiotics resulting in more illness, healthcare costs, and deaths. Their cell envelope consists of the inner membrane (IM) surrounding the cytoplasm and an outer membrane (OM) that protects the cells from harsh conditions. The OM is an asymmetric bilayer made up of phospholipids (PL) and lipopolysaccharides (LPS). Also, the OM harbors numerous β- barrel proteins (outer membrane proteins, OMPs). Both LPS and OMPs are synthesized in the cytoplasm and subsequently transported across the periplasm into the OM. In E. coli, the β-Barrel Assembly Machinery (BAM) complex, which consists of the BamABCDE subunits mediates folding and insertion of β-barrel proteins from the periplasm into the OM. Similarly, the Lpt system consiting of seven essential proteins LptABCDEFG spanning the entire cell envelope form the LPS transport system.
3.1 The BAM complex - β-barrel folding and insertion in the native membranes
As the asymmetric outer membrane is an integral part of the BAM complex, mechanistic investigations must ideally be performed in whole cells or native membranes. Using the in situ ESR spectroscopy approach, which we demonstrated over the past years, we study the interaction between BAM and the chaperone-protein complex and how the conformational changes in BAM are coupled to protein folding and insertion.
Related Publications:
- Gopinath A, Rath T, Morgner N, Joseph B. Lateral gating mechanism and plasticity of the BAM complex in micelles and E. coli. PNAS Nexus, 3, pgae019, https://doi.org/10.1093/pnasnexus/pgae019
- Gopinath A and Joseph B (2021) Conformational flexibility of the protein insertase BamA in the native asymmetric bilayer elucidated with ESR spectroscopy. Angew. Chem. Int. Ed., e202113448, https://doi.org/10.1002/anie.202113448
3.2 The Lpt System - dynamic basis for lipopolysaccharide (LPS) transport and regulation
In E. coli, the Lpt system transports LPS molecules from the inner membrane to the outer membrane through the tans-periplasmic bridge consisting of LptC, LptA, and the N-terminal domain of LptD. The ABC exporter LptB2FG located in the inner membrane transfers LPS molecule to LptC. Subsequently, the LPS moves along the periplasmic bridge and finally LptDE inserts it into the OM. How the individual modules dynamically interact and achieves a huge efficiency to transports millions of LPS molecules during each lifecycle remain elusive. We use membrane reconstitution, orthogonal labels, and time resolved ESR spectroscopy to elucidate the transenvelope communication underlying LPS transport and regulation.
Related Publciations:
- Dajka M, Rath T, Morgner N and Joseph B. Dynamic basis of lipopolysaccharide export by LptB2FGC. eLife https://doi.org/10.7554/eLife.99338.2