Physics Colloquium: Prof. Dr. Peter Vöhringer: Emit a greenhouse gas and learn about its binding: Insights from femtosecond infrared spectroscopy
Prof. Dr. Peter Vöhringer
Image Credit: Universität Bonn
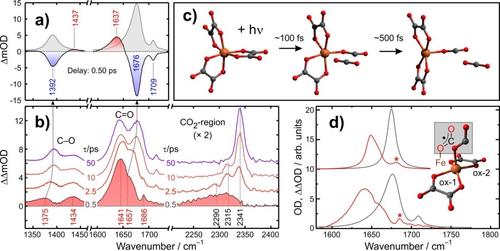
Fig. 1
Image Credit: Prof. Dr. Peter Vöhringer
Universität Bonn, Clausius Institute for Physical and Theoretical Chemistry, Molecular Physical Chemistry
The utilization of CO₂ as an abundant, renewable carbon source for a sustainable synthesis of fine chemicals represents an intriguing strategy that may assist in the future to reduce anthropogenic greenhouse gas emissions. Following nature’s examples of CO₂-fixation such an approach can rest on the activation of the inert gas by a suitable metal; specifically, an earth-abundant 3d-transition metal (TM) for sustainability reasons.
In the past, inorganic chemists have successfully prepared a number of different TM-complexes bearing a CO₂-ligand. Formally, these compounds can be classified according to the mode of CO₂-binding to the TM: (i) the η¹(C)-mode with σ-bonding between the metal and the central C-atom, (ii) the η²(C,O)-mode (or “side-on” coordination) with π-bonding between the TM and one of the two C=O bonds, and (iii) the η¹(O)-mode (or “end-on” coordination) with σ-bonding between the metal and a terminal O-atoms.
Such ingenious research efforts notwithstanding, a profound understanding the electronic-structural factors that govern CO₂-TM binding is still lacking. Here, we utilize femtosecond infrared spectroscopy in combination with density functional theory learn about these fundamental principles. To this end, we study photochemical precursors bearing the photolabile oxalate ligand, C₂O₄²─. Upon impulsive optical excitation, such systems release one molecule of CO₂ and retain a dianionic carbonite (CO₂²─) ligand. We will discuss the ultrafast electronic structural reorganization dynamics that ultimately yield the sought CO₂-TM complex and provide design guides for steering it toward a desired binding motif.
Time & Location
May 24, 2024 | 03:00 PM c.t.
Lecture Hall A (1.3.14)
Department of Physics
Arnimallee 14
14195 Berlin
Further Information
Host
Keywords
- CO₂
- CO₂-TM binding
- colloquium
- density functional theory
- earth-abundant 3d-transition metal
- emissions
- femtosecond infrared spectroscopy
- greenhouse gas
- Peter Vöhringer
- photochemical precursors
- photolabile oxalate ligand
- physics
- research
- spectroscopy
- TM
- ultrafast electronic structural reorganization dynamics